The first research on ampreometric determination of blood glucose using a redox couple-mediated, glucose
oxidase (GOx)-catalyzed reaction was demonstrated by Williams
et al. (1970). But this study did not lead to the rapid application of amperometry in self-monitoring blood glucose in the
home. The first electrochemical blood glucose monitor for selfmonitoring diabetic patients was pen-sized and was launched in
1987 as ExacTech by Medisense Inc. Many studies on the development of blood glucose sensors have been carried out since the
1980s
Most glucose sensors use enzyme based detection where the first one is Glucose oxidase(GOD) and the second one is Glucose dehydrogenase(GDH).
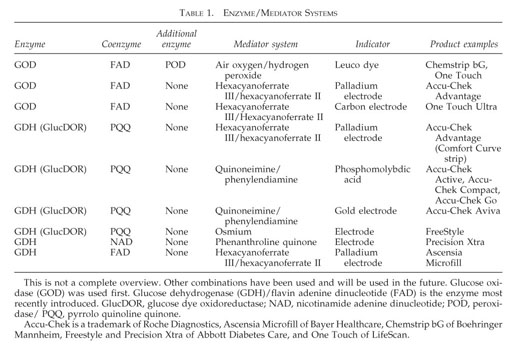
Contents of Glucose Strip
- GOD or GDH Enzyme
- Cofactor/Coenzyme
- enzyme
- glucose dehydrogenase pyrroloquinoline quinone (GDH-PQQ)
- Mediator (less than 300mg) potassium hexacyanoferrate (III), phenazine methosulfate, phenothiazine, and hexaammineruthenium (III) chloride) (ref 3)
- Buffer
- Other Non Reactive ingredients Carboxy Methyl Cellulose (CMC Powder), Aerosile (SiO2), Alginate, PEG
- Beta-hydroxybutyrate-dehydrogenase: An enzyme that can be used to detect diabetic ketoacidosis (DKA)
Numerous organic and inorganic compounds were proposed as mediators for biosensors: ferricyanide, ferrocene, phenazine, phenothiazine, methylene green/blue, tetrathiafulvalene, quinone, osmium and ruthenium complexes. However, ferricyanide is still the most used mediator in commercial glucose biosensing. Hexaammineruthenium (III) is an interesting candidate because the hexaammineruthenium (II/III) redox potential is lower than the hexacyanoferrate (II/III) one. The low working potential leads to low interferences and increase in selectivity and accuracy. (ref 4)
K3[Fe(CN)6], [Ru(NH3)6]Cl3, d-glucose, potassium l-lactate, chitosan, phenazine methosulfate (PMS), phenothiazine, (3-aminopropyl)triethoxysilane (APTES), Triton X-100 (C9H19C6H4(OCH2CH2)9OH) and glucose oxidase (EC 1.1.3.4, 248 IU mg−1) from Aspergillus niger (ref 3)
Ag-reference electrode were produced using silver polymer paste (NPP “Delta-Paste”, Zelenograd, Russia), carbon paste (C2030519P4, Sun Chemical, South Normanton, UK) and UV curable insulating paste (UNICA, Ternat, Belgium). (ref 4)
 |
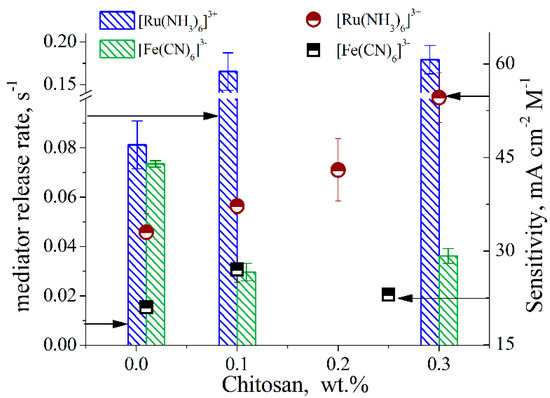
| mediator release rate from chitosan matrix affecting current(ref 4) |
Figure 4. Release rates of [Ru(NH3)6]3+ and [Fe(CN)6]3− (blue and green bars, respectively) from chitosan membranes (formed by drop-casting the mediator in polymer solutions on the electrode) and sensitivity of the glucose test strips based on GOx (10 mg Ml−1) and 100 mM [Ru(NH3)6]3+ or [Fe(CN)6]3− (red and black dots, respectively), 50 mM K2HPO4/KH2PO4, 180 mM NaCl, pH 7.4 (ref 4)
Key Factors in Functional Layer mixture
- Enzyme & Coenzyme selection
- Mediator Selection (cost, efficiency and interference with polymer or blood components)
- Selection of immobilisation method or polymers
- Effect of conc. of mediator, polymer and enzyme co enzyme(should able analyse amperometry/chronoamperometry plot data to change in conc. of components)
- Analyse properly when measured values are not linear to glucose conc.
Key factors of Electrode Design
- Size/Area of Reference Electrode
- Distance between Reference and working electode
- Coating on working electrode
- Ink/extra layer of Reference electrode (reduction potential)
- Positioning of blood detect electrode
- Conductivity of ink traces (should be less than 1.5K ohms for total path)
- max current 80uA at 250mV (less than 200mV is good as no interference from other components present in whole blood)
- Extra selective layer requirement (for filtering ascorbic acid etc.)
Key factors in instrumentation
- Incubation time
- Coulometry or amperometry
- If amperometry then when to record current (peak or plateu zone)
References
1.The Impact of the Functional Layer Composition of Glucose Test-Strips on the Stability of Electrochemical Response. Chemosensors 2022, 10, 298. https://doi.org/10.3390/chemosensors10080298 (stabilty & content)
2. Heinemann, Lutz. (2010). Quality of Glucose Measurement with Blood Glucose Meters at the Point-of-Care: Relevance of Interfering Factors. Diabetes technology & therapeutics. 12. 847-57. 10.1089/dia.2010.0076.
3. The widest linear range of glucose test strips based on various mediators and membranes for whole blood analysis,https://doi.org/10.1016/j.jelechem.2023.117445. (polymer)
4. Electrochemistry of Freely Diffusing Mediators in Polyelectrolyte Membranes Used for Blood Glucose Test Strips with a High Upper Limit of the Linear Range. Engineering Proceedings. 2023; 35(1):19. https://doi.org/10.3390/IECB2023-14603
6. Glucose test strips with the largest linear range made via single step modification by glucose oxidase-hexacyanoferrate-chitosan mixture,
Biosensors and Bioelectronics,https://doi.org/10.1016/j.bios.2022.114851. (largest linear range with chitosan)
7. Hu, Jamie. (2008). The evolution of commercialized glucose sensors in China. Biosensors & bioelectronics. 24. 1083-9. 10.1016/j.bios.2008.08.051. (direct printed all in one paste print & strip design)
8. https://www.basinc.com/assets/library/presentations/pdf/JOH-01.pdf
9. https://en.wikipedia.org/wiki/Cottrell_equation
10. https://en.wikipedia.org/wiki/Faradaic_current
11. https://en.wikipedia.org/wiki/Anson_equation
12. https://www.thno.org/v12p0493.htm
13. https://en.wikipedia.org/wiki/Coffee_ring_effect
14. Portable optical biosensor for point-of-care monitoring of salivary glucose using a paper-based microfluidic strip, https://doi.org/10.1016/j.biosx.2024.100452. (immobiliser)
15. Mediator Preference of Two Different FAD-Dependent Glucose Dehydrogenases Employed in Disposable Enzyme Glucose Sensors. Sensors 2017, 17, 2636. https://doi.org/10.3390/s17112636









Comments
Post a Comment